Don't hesitate to send a message
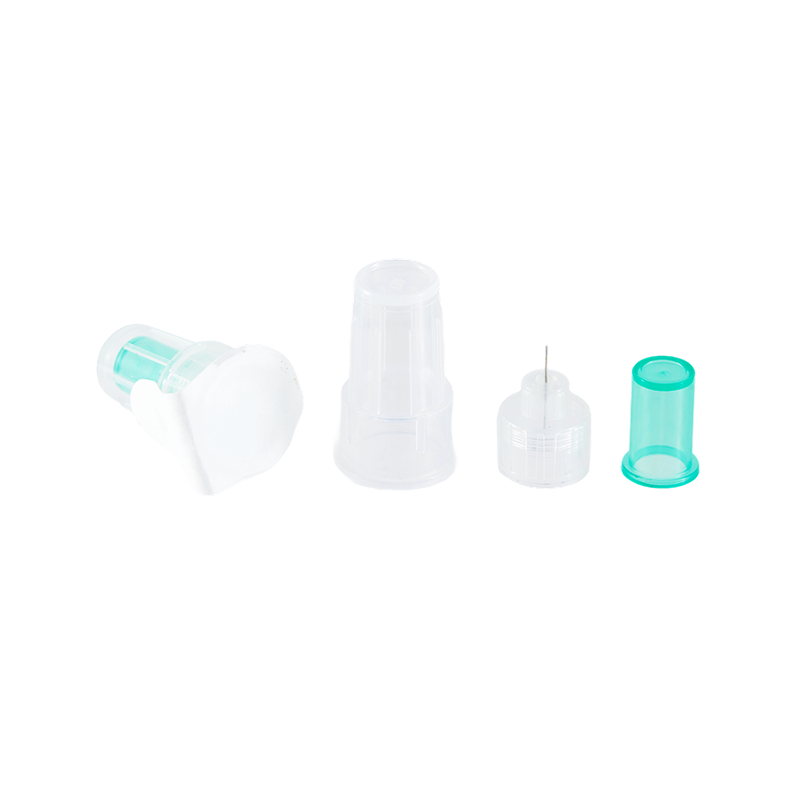
The design and production comply with ISO8537. The plastic parts are moulded by ...
When it comes to insulin delivery systems, especially Needle Tips For Insulin Pens, Needle Caps For Insulin Pens, and Insulin Pen Needle Caps, the packaging process must meet strict standards to maintain sterility, functionality, and safety during transportation and storage. Proper packaging ensures that these small yet essential accessories reach healthcare facilities and patients in perfect condition, ready for safe and reliable use.
Packaging for Needle Tips For Insulin Pens is more than just an aesthetic or logistical concern—it directly influences the safety and quality of the product. Since these components come into contact with medical devices and are used for subcutaneous injections, they must remain free from contamination. Manufacturers design the packaging to protect against moisture, dust, and accidental damage during shipping.
Similarly, Needle Caps For Insulin Pens require specialized packaging that maintains their structural integrity. Even slight deformation or cracks can compromise their ability to fit securely on insulin pens. The packaging also helps prevent exposure to environmental factors such as temperature fluctuations or humidity, both of which could affect the material properties.
Insulin Pen Needle Caps, being a key component that covers the needle tip, are packaged to ensure complete sterility until the moment of use. This not only supports product quality but also complies with global medical packaging standards and regulatory requirements.
The packaging materials chosen for Needle Tips For Insulin Pens must balance durability and lightweight construction. Commonly used materials include medical-grade paper, polyethylene films, and blister packs with easy-peel features. These materials protect the product while allowing healthcare professionals or patients to open them effortlessly.
For Needle Caps For Insulin Pens, packaging design must prevent any friction or compression that could alter the cap’s shape. Manufacturers often use thermoformed trays that securely hold each cap in place, reducing movement during transit. The packaging is then sealed with sterile film to preserve cleanliness.
In the case of Insulin Pen Needle Caps, labeling is another critical aspect. Each package includes details such as batch numbers, manufacturing dates, and usage instructions. Clear labeling helps trace product origins and ensures proper inventory management for hospitals, pharmacies, and distribution networks.
Maintaining sterility is the highest priority in packaging Needle Tips For Insulin Pens. The entire process—from molding to sealing—takes place in a controlled cleanroom environment. Automated packaging lines minimize human contact, reducing the possibility of contamination.
The Needle Caps For Insulin Pens undergo similar procedures. They are sterilized using methods such as ethylene oxide (EO) gas before packaging, then sealed immediately to retain sterility until opened. Each package is tested to ensure the seal’s integrity, guaranteeing that the Insulin Pen Needle Caps remain uncontaminated throughout their shelf life.
Additionally, all packaging materials must meet ISO and FDA requirements for medical devices. This ensures compatibility between the packaging and the components, preventing chemical interaction or leaching that could compromise product safety.
For patients and medical staff, ease of use is a vital aspect of packaging design. Needle Tips For Insulin Pens are often packed in individual sterile blister packs that are simple to open, even for individuals with limited dexterity. The design allows users to remove the product without touching the sterile areas, maintaining cleanliness during handling.
When it comes to Needle Caps For Insulin Pens, packaging must support fast identification and retrieval. Multi-pack configurations are commonly used in clinics, while compact packs are designed for personal or travel use. This ensures that Insulin Pen Needle Caps remain accessible and organized for daily insulin administration routines.
Manufacturers also consider sustainability by using recyclable materials and optimizing packaging size to reduce waste. As environmental awareness grows, eco-friendly packaging options are becoming increasingly important for medical device companies.
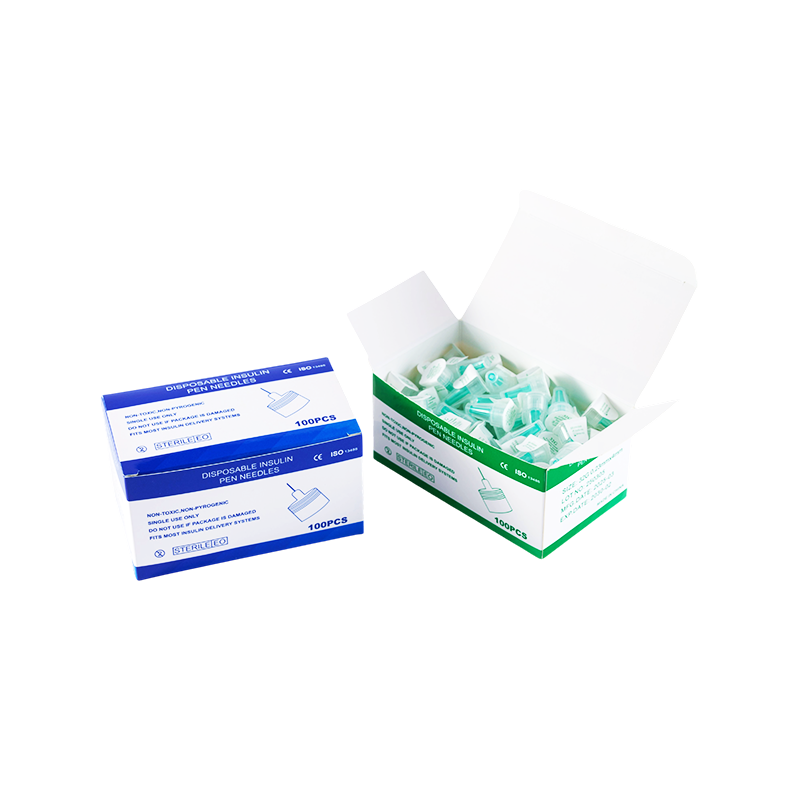
Proper packaging also ensures that Needle Tips For Insulin Pens, Needle Caps For Insulin Pens, and Insulin Pen Needle Caps can withstand long-distance transportation without damage. Packages are designed to resist vibration, impact, and pressure changes, especially for international shipments.
Storage instructions are clearly marked on each package, indicating recommended temperature and humidity levels. Warehouses and distributors follow these guidelines to ensure the Needle Tips For Insulin Pens and related accessories maintain their quality until they reach end users. The same applies to Needle Caps For Insulin Pens and Insulin Pen Needle Caps, whose material properties could degrade under improper conditions.
The design and production comply with ISO8537. The plastic parts are moulded by ...

There are three kinds of the tip types, Sealed-circle with 2 side holes, sealed-...

Assembling with insulin pen, for insulin hypodermic injection.The plastic parts ...

Used in conjunction with an insulin pen, it is used for subcutaneous injection o...

The cannula is made of high quality austenite stainless steel.All the components...
The material of the needle is Medical grade SUS304,which have great stiffness, t...
The barrel is made from high transparent polypropylene(PP),which have a bright a...

The cannula is made of high quality austenite stainless steel.The lancet tip is ...