Don't hesitate to send a message
The design and production comply with ISO8537. The plastic parts are moulded by ...
Insulin delivery devices are part of everyday routines for millions of people managing diabetes. Among them, the Insulin Pen Needle stands out as a small but essential component that directly affects user experience and dosing reliability. From a manufacturing perspective, this product requires a balance between precision engineering, stable materials, and repeatable production processes.
Healthcare providers and distributors often focus on consistency, compatibility, and reliable supply rather than marketing language. These practical concerns shape how factories like ours approach product development and long-term production planning.

People who rely on insulin pens expect injections to feel predictable and easy to perform. Inconsistent needle sharpness, uneven wall thickness, or unstable hub connections can interrupt that routine. Even minor variations may affect how smoothly insulin flows or how the needle penetrates the skin.
For the Diabetes Care industry, addressing these pain points begins at the production stage. The design of an Insulin Pen Needle must align with standard pen systems while maintaining dimensional accuracy across large volumes. Factories must focus on repeatability rather than one-off performance.
From an engineering viewpoint, the structure of an Insulin Pen Needle is straightforward but demanding. The stainless steel cannula is drawn to a fine diameter, cut to length, and ground into a multi-bevel tip. This process requires controlled tooling and polishing steps to maintain a consistent surface finish.
The plastic hub, usually made from medical-grade polymers, must provide a stable connection to insulin pens without deformation. Material selection plays a direct role in thread compatibility and resistance to stress during use. These design decisions are not visible to end users, yet they strongly influence performance.
In a factory environment, Insulin Pen Needle production follows a standardized workflow. Raw stainless steel tubing is inspected for diameter tolerance and surface quality before processing. Automated grinding machines shape the needle tip, followed by cleaning and surface treatment.
Assembly takes place in cleanroom conditions to reduce contamination risk. Each needle is fixed into its hub through controlled bonding methods, then inspected for alignment and attachment strength. Throughout production, inline inspection systems monitor dimensional stability and appearance.
This level of control allows Insulin Pen Needle Suppliers to maintain stable output across long production cycles without unexpected variation.
Medical injection components must comply with recognized international standards related to dimensions, sterility, and biocompatibility. For an Insulin Pen Needle, this means regular sampling tests, penetration force checks, and flow performance evaluations.
Traceability is another key requirement. Lot numbers, production dates, and material records allow issues to be tracked efficiently if questions arise in the supply chain. These systems support long-term cooperation within the Diabetes Care sector.
By focusing on consistent production processes, cleanroom assembly, and practical compatibility with insulin pens, factories support reliable daily use without unnecessary complexity. Behind the small size of each needle is a system built around precision, traceability, and long-term cooperation within the healthcare supply chain.

The design and production comply with ISO8537. The plastic parts are moulded by ...

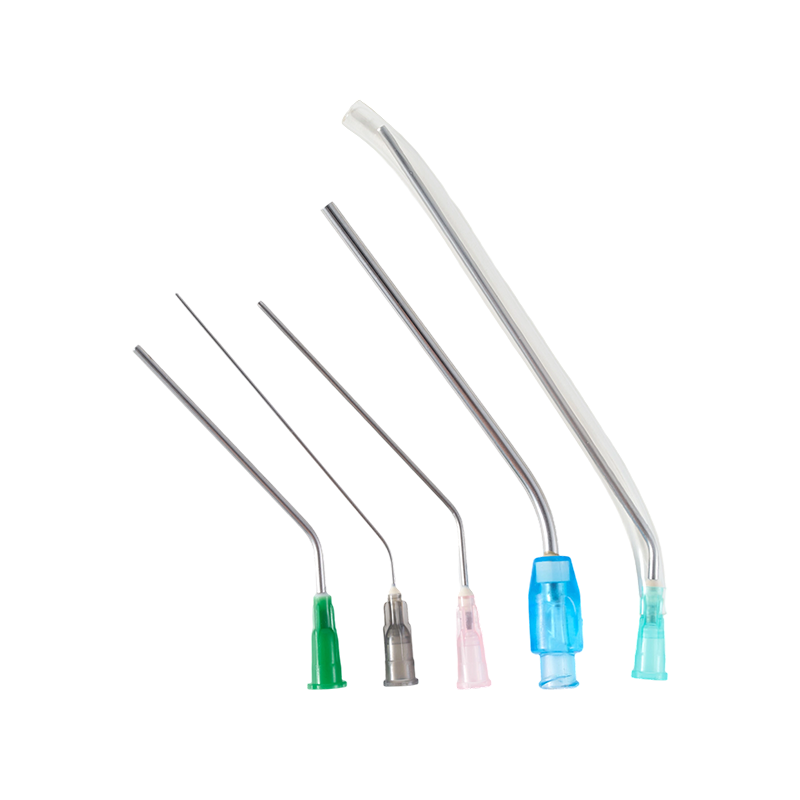
There are three kinds of the tip types, Sealed-circle with 2 side holes, sealed-...

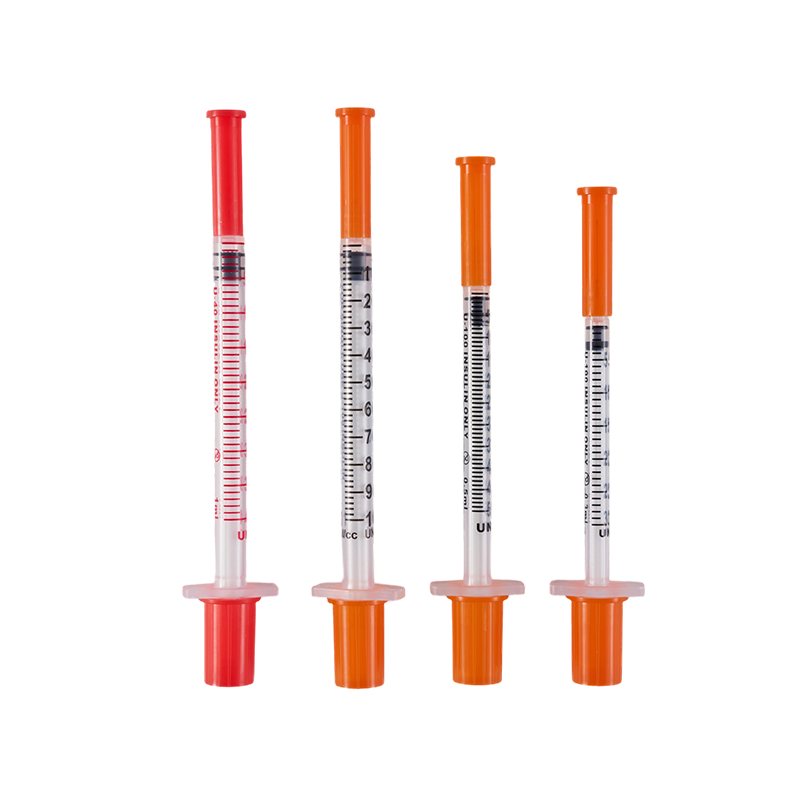
Assembling with insulin pen, for insulin hypodermic injection.The plastic parts ...

Used in conjunction with an insulin pen, it is used for subcutaneous injection o...

The cannula is made of high quality austenite stainless steel.All the components...
The material of the needle is Medical grade SUS304,which have great stiffness, t...
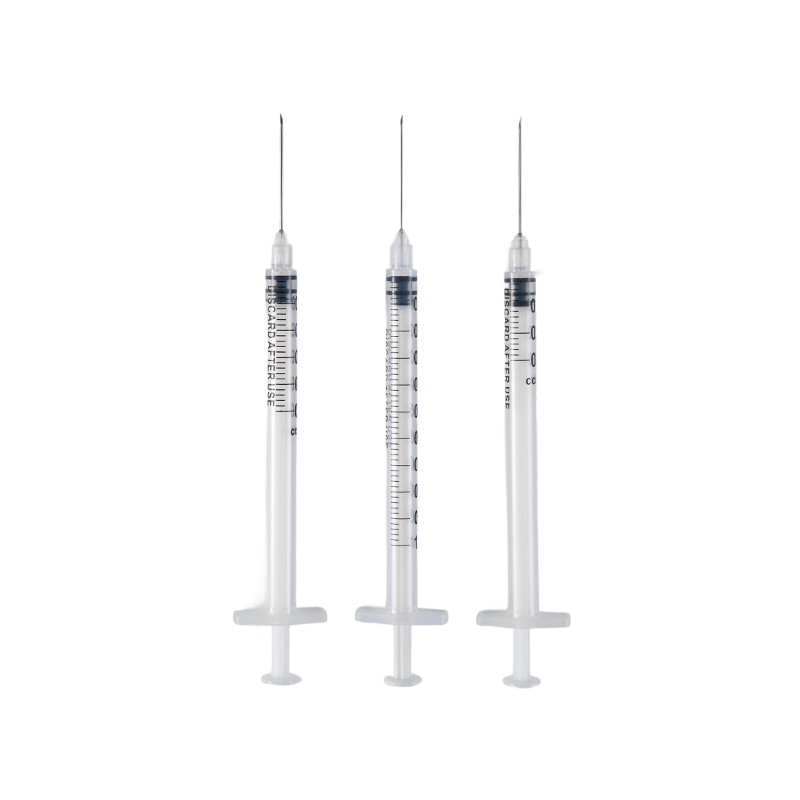
The barrel is made from high transparent polypropylene(PP),which have a bright a...

The cannula is made of high quality austenite stainless steel.The lancet tip is ...